|
|
 |
 |
 |
 |
 |
|
 |
|
Frequently Asked Questions About Generics |
|
|
What are generic drugs?
If generic drugs are the same as brand drugs, why are they sometimes
different shapes, sizes and colors?
What kind of savings can generics generate for American consumers?
How are generic companies able to offer a product that is much less
expensive than the brand product?
Generic drugs may save consumers money, but are they as safe and
effective as their brand counterparts?
Do brand companies have more modern manufacturing facilities than
generic companies?
Why isn’t there a generic version available for all brand drugs?
Importation seems to be a perfect answer to lowering prescription drug
costs. Why do the brand and generic pharmaceutical industries oppose
this initiative?
When should a generic drug not be substituted for the brand name drug?
Who can the consumer turn to for more information about the benefits
of generic products? |
| |
What are
generic drugs?
Generic drugs are versions of brand drugs whose patent terms have
expired. Generics are referred to by their chemical or generic name.
For example, fluoxetine is the generic name for Prozac. |
| |
If generic drugs are the same as brand drugs, why are they sometimes
different shapes, sizes and colors?
All pharmaceutical products, whether brand or generic, vary slightly.
Sometimes a brand company patents the appearance of the drug or the
brand drug may be protected by trademark or common law. This may
prohibit a generic manufacturer from adopting the appearance of the
brand drug. However, the same testing that assures batch to batch
consistency for a brand product is used to ensure equivalence of
generics. Any cosmetic differences between the generic and the brand
product in no way impacts the sameness or safety of the generic
version. |
| |
What kind of savings can generics generate for American consumers?
Generic pharmaceuticals can cost 30 - 80 percent less than the
equivalent brand product. For every healthcare dollar spent on
medicines, American consumers spend less than eight cents on generic
drugs versus 92 cents on brand drugs. This is despite the fact that
generic drugs are dispensed for nearly half of all prescriptions. |
| |
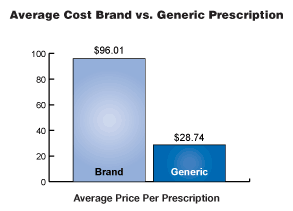
| In 2004,
the average price of a prescription filled with a generic drug was
$28.74 and the average price of a prescription dispensed with a brand
drug was $96.01. When substituting an equally effective generic drug
for the brand product, American consumers saved an average of
approximately 70 percent. With healthcare costs rising exponentially,
that is a savings every American can use. Generics offer these savings
without sacrificing quality, safety or effectiveness. |
| |
 |
| |
How are companies providing generic products able to offer a product
that is much less expensive than the brand product?
Generic companies spend little money on marketing generic products.
Because there are often multiple generic competitors for each product,
and because generics are sold under the same chemical name, what
limited advertising and marketing activities occur are used to ensure
that trade customers (drug store chains, wholesalers and pharmacies)
are aware that a generic is available.
In addition, the expense of developing a
generic product is much lower than the typical expense of developing a
brand product. This is why brand companies receive market exclusivity;
it ensures that they can recoup their investment in new product
development.
In order to market a generic
equivalent to a brand pharmaceutical company’s drug product (once the
market exclusivity on the innovator's product has expired), a generic
pharmaceutical company uses the Abbreviated New Drug Application (ANDA)
process of the Food and Drug Administration (FDA).
Under this process, the generic
manufacturer uses the safety and efficacy data supplied by the brand
company, and must only prove to the FDA that its generic product is
equivalent to the branded product. |
| |
Generic drugs may save consumers money, but are they as safe and
effective as their brand counterparts?
Absolutely. To be approved, a generic must have the same active
ingredients, same dosage form, same standards for purity and quality,
same standards for manufacturing and same clinical effect as the brand
product. As far as safety, the FDA requires all drug manufacturers and
their facilities to adhere to specific guidelines, called current Good
Manufacturing Practices (cGMP), no matter what the drug and no matter
who the manufacturer. |
| |
 Do
brand companies have more modern manufacturing facilities than generic
companies? Do
brand companies have more modern manufacturing facilities than generic
companies?
No. Generic companies have facilities comparable to brand firms. The
FDA requires both brand and generic companies to conform to the same
high manufacturing standards, known as current Good Manufacturing
Practices (cGMPs). To ensure compliance with these standards, the FDA
makes nearly 3,500 inspections a year of both brand and generic
companies.
Why isn’t there a generic version available for all brand drugs?
When brand companies develop a new drug, they are granted
patent protection or another form of exclusivity for up to 20 years.
The patent protects the drug’s research, development and marketing
activities. When the patent expires, drug companies may introduce
generic versions but only after they are thoroughly tested by the
manufacturer and approved by the FDA. |
| |
Importation seems to be a perfect answer to lowering prescription drug
costs. Why do the brand and generic pharmaceutical industries oppose
this initiative?
The generic pharmaceutical industry opposes importation for several
reasons. First, data used to support importation considers only a
handful of top-selling brand name drugs and exaggerates the potential
savings. A more comprehensive representative sample of drugs purchased
by U.S. consumers found that average prices in Canada, Germany, Sweden
and Switzerland were comparable to or higher than those when U.S.
generic drugs are included in the comparison.
The quality of America’s prescription
medicines is the highest in the world. Importation would significantly
undermine this quality standard. There is no mechanism for assuring
whether imported drugs meet basic quality standards or whether they
are expired, sub-potent, properly labeled, contaminated or
counterfeit. Importation places consumers in the dangerous position of
“buyer beware” without any mechanism to protect their safety.
If importation is allowed, consumers
will be able to import medicines of questionable quality, with no
restrictions on the amount or frequency of the importation. Foreign
pharmacies and wholesalers will not be subject to FDA or state
oversight, there will be no assurance of appropriate drug handing, and
no ability to prevent shipment of counterfeit or tainted drug products
directly to consumers.
When should a generic drug not be substituted for the brand name drug?
The simple answer to this question is: Never.
FDA-approved generic drugs that are rated bioequivalent (AB-Rated) to
the brand drug must prove that they offer the same safety and
effectiveness as the brand name prescription drug. Although some
groups would suggest that certain drugs, such as mental health drugs,
should not be substituted, there is no scientific or clinical evidence
to suggest that the generic will not offer the same benefits as the
brand drug, at a substantially lower cost to the patient.
Who can the consumer turn to for more information about the benefits
of generic products?
The pharmacist plays a key role in explaining the quality, medicinal
comparability and financial benefits offered by generic products.
Recent surveys show that when consumers discuss generic
pharmaceuticals with their pharmacists, the overwhelming majority
conclude that generic products represent an important healthcare
alternative, one that is as safe and effective as the branded product.
Physicians can also help consumers decide if a generic alternative is
available for treating their illness.
The FDA is also an excellent resource
for information about generic drugs. Visit the web site at
www.fda.gov/cder/ogd/index.htm.
|
| |
 |
 |
 |
 |
 |
|
|
| | |